Background
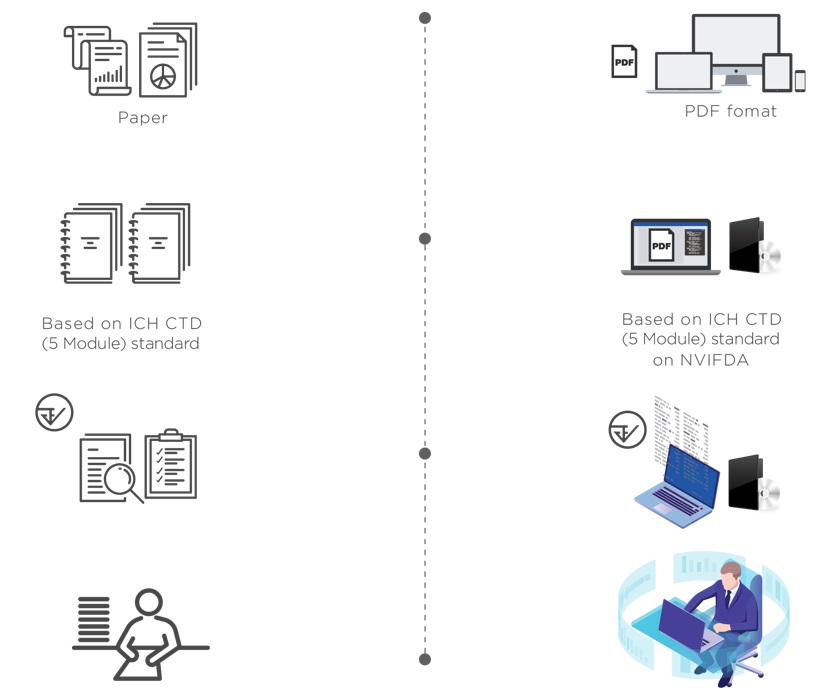
NVIFDA is an eCTD submission management software with the collaboration of National Vaccine Institute (NVI) and Thai Food and Drug Administration (Thai FDA) in order to transform pharmaceutical product registration process from paper-based to electronics-based. NVIFDA has been developing since 2015 with the collaboration of government authorities and pharmaceutical companies.
About electronics submission